This first-ever submission is the culmination of twelve years of research data and four PRF-funded clinical trials in partnership with Brown University and Boston Children’s Hospital, and made possible by the courageous children and their families, as well as YOU – PRF’s wonderful community of donors.
You can read more about this exciting news यहाँ.
Eiger BioPharmaceuticals will submit completed portions of the application for FDA review on an ongoing basis, with plans to finish the submission in the first quarter of the new year. Approval will enable these children and young adults to access lonafarnib – which has been shown to give them stronger hearts and longer lives – by prescription, instead of through our clinical trials, in the US and possibly in other countries as well.
What a positive way to end 2019, and start the New Year strong! We have worked tirelessly to make a meaningful impact on the lives of children and young adults with Progeria, and this submission brings us closer to that goal.
THANK YOU ALL for supporting the research that not only has brought us to this pivotal point, but also allows us to continue working to discover new drugs that will ultimately cure these extraordinary children.
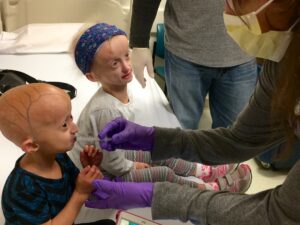
Zoey and Carly getting their lonafarnib treatment during their latest clinical trial visit to Boston.
