News
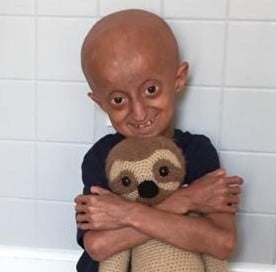
Exciting breakthroughs in RNA Therapeutics for Progeria!
We’re thrilled to share the results from two very exciting breakthrough studies on the use of RNA therapeutics in Progeria research. Both studies were co-funded by The Progeria Research Foundation (PRF) and co-authored by PRF’s Medical Director, Dr. Leslie Gordon.

Kicking off the New Year with exciting research news!
In January, the science journal Nature published breakthrough results demonstrating that genetic editing in a mouse model of Progeria corrected the mutation that causes Progeria in many cells, improved several key disease symptoms and dramatically increased lifespan in the mice.

The day has come: FDA approval for first-ever Progeria treatment!
We’re thrilled to announce that today, we achieved an important piece of PRF’s mission: lonafarnib, the first-ever treatment for Progeria, has been granted FDA approval.
PRF’s 10th International Scientific Workshop
Enjoy some clips, photos, comments, and Q&A!

The COUNTDOWN to PRF’s VIRTUAL Soar to the Cure Gala has begun!
Join us on Dec. 5, 2020 for another epic Night of Wonder, this year from the comfort of your own home!

PRF’s 2020 Newsletter!
Read about how our progress continues despite the pandemic; the science driving PRF’s latest grant recipients to propel Progeria research to new heights; inspiring thoughts from families in our Progeria community; and so much more.

July 20: PRF’s Annual ONEpossible Campaign a Success!
From all of us at PRF, as well as the children and their families, THANK YOU to everyone who donated to our ONEpossible campaign!!
We CRUSHED our goal – which will help to offset funds lost from cancelled events due to COVID-19 – and couldn’t have done it without your support!

A Note to our PRF Community
First and foremost, we hope you are staying well. In light of the recent progression of COVID-19, and as we all navigate this uncertain time, we want to THANK YOU for your support and let you know that our fight against Progeria remains steadfast.

Application to FDA for lonafarnib approval is COMPLETE!
During an otherwise difficult time for our world, we are happy to share a bright spot: Eiger BioPharmaceuticals has completed submission of a New Drug Application (NDA), seeking approval – in Europe and the US – of the drug lonafarnib as a first-ever treatment for Progeria.

Thank you for helping us celebrate Meghan’s 19th birthday this month!
On March 1st, PRF Ambassador Meghan Waldron turned 19, and we celebrated with March Madness 2020: Where in the world is Meghan Waldron? We've shared photos from Meghan’s travels and hope they've inspired you to think about where your next adventure will be once we're...

